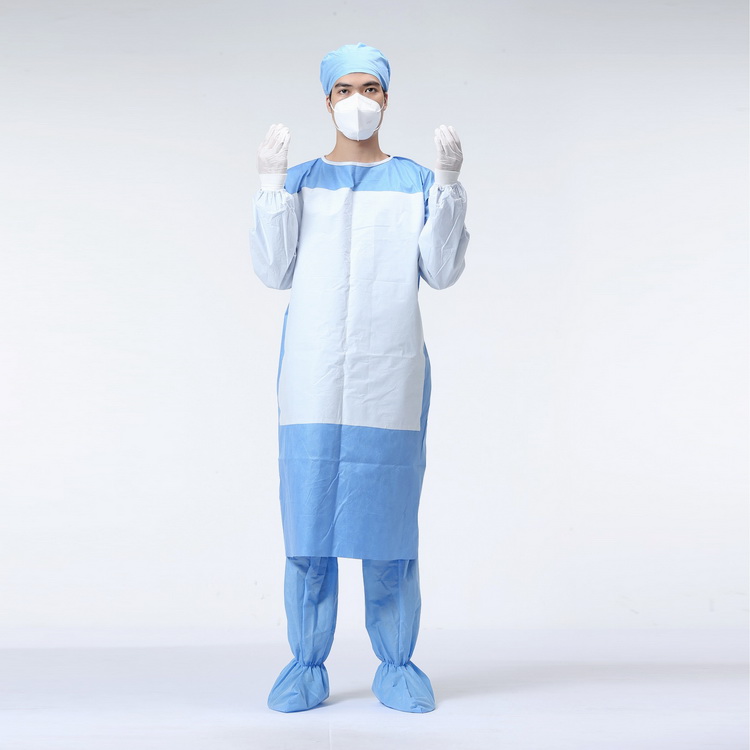
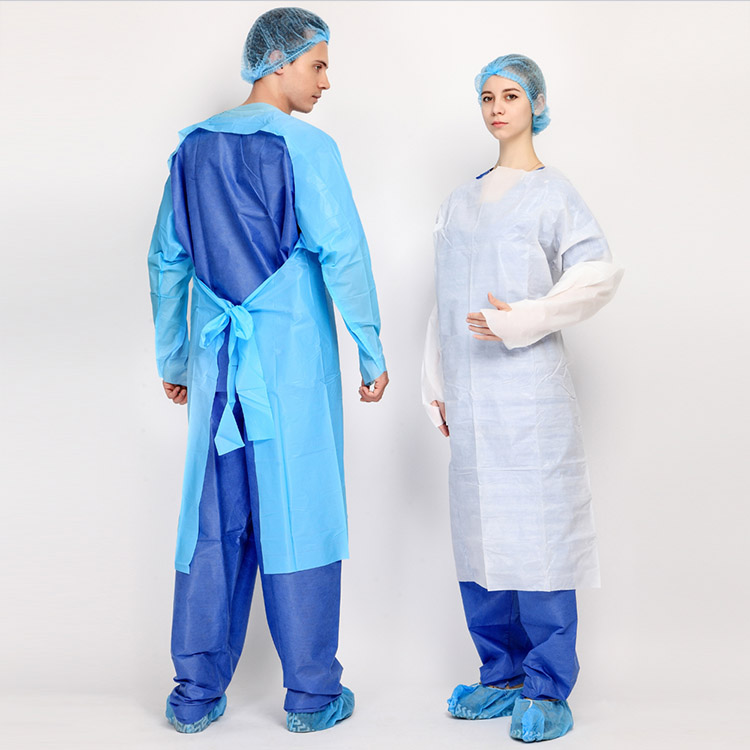
Round neck brushed hand pants
Parameter specifications
| Product Name | Round neck brushed hand pants |
| Raw material name | SMMS and spunlaced fabric |
| Product size | Customized according to clinical needs |
| Packing quantity | Customized according to clinical needs |
| Product Usage Scope | Suitable for medical staff entering the operating room to wear and prevent liquid splashing during surgery |
| Medical Device Production License Number | Zhejiang Food and Drug Administration Production License No. 20130177 |
| Medical Device Registration Certificate Number | 20152140476 |
| Product technical requirement number | 20152140476 |
| performance | The surgical gown is sterilized with ethylene oxide, and the residual amount of ethylene oxide at the factory should not exceed 10 μ G/g. The product has been sterilized. |
Storage and transportation conditions:
The packaged surgical gowns should be stored indoors with a relative humidity of no more than 80%, free from corrosive gases, dry, well ventilated, and clean. Transportation shall be carried out using general means of transportation or in accordance with the provisions of the supply contract.
Notes and other prompts:
1. Before use, please pay attention to the sterilization label and expiration date on the packaging bag. If there is no sterilization label or the expiration date has been exceeded, it is strictly prohibited to use.
2. This surgical gown is a disposable product and cannot be reused.3. Before opening the packaging bag, please visually inspect it for any damage. If there is any damage, it cannot be used.
4. When wearing, please extend both hands directly into the sleeve and then open both arms to support the clothes, so that the surgical gown can be worn naturally on the body. Remember not to touch the outer side of the surgical gown with both hands, and have a nurse help fasten the belt.
5. If you find any damage or belt detachment in the surgical gown after wearing it, please do not use it again.
6. This product should avoid contact with flames and extremely hot object sources to avoid combustion.
7. When disposing of this product after use, it should be strictly handled in accordance with the requirements of the medical unit.
8.This product is sterilized with ethylene oxide and has a validity period of three years
Product inquiry